Sulfur dioxide reacts with oxygen to form A SO3. A if 34 moles of sulfur dioxide reacts with oxygen gas how many moles of sulfur trioxide will form.

Solved Sulfur Dioxide So 2 Reacts With Oxygen O 2 To Chegg Com
See answer 1 Best Answer.

. 2SO₂g O₂g 2SO₃g And Kp is defined as. Sp2 to sp3 sp2 to sp sp3 to sp3d sp2 to sp3d2 No change takes place. Sulfur trioxide SO3 is a chemical compound that is a significant pollutant in gaseous form as it is involved in the production of acid rain.
700 atmI know the answer is 600 but I dont know how that is the answer. Sulfur dioxide reacts with oxygen to form sulfur trioxide2 SO2 g O2 g --. Stage two making sulfur trioxide.
What change in hybridization of the sulfur occurs in this reaction. If you add in the first 059atm of SO₂ and 29atm of O₂ the equilibrium pressures will be. Sulfur dioxide and oxygen react to form sulfur trioxide during one of the key steps in sulfuric acid synthesis.
Sulfur dioxide reacts with Oxygen to form Sulfur trioxide. Sulfur dioxide with oxygen. B how many moles of oxygen will react completely with 47 moles of sulfur dioxide 02 t 2502 ⑨ 34m01 502 ④ 47m01 402 3.
P SO₂ 059atm - 2X. For 53 mole SO2 mole of SO3 produced 11 x 53 53 mol 3. Sulfur or sulphur in British English is a chemical element with the symbol S and atomic number 16.
P O₂ 29atm - X. 35m01 02 ⑤. 2SO2g O2g 2SO3g In a 100 L flask 200 atm of SO2 reacts wit.
2SO 2g O 2g 2SO 3 g The industrial conditions for the chemical reaction are 1 The Temperature is 450 C. It is abundant multivalent and nonmetallicUnder normal conditions sulfur atoms form cyclic octatomic molecules with a chemical formula S 8Elemental sulfur is a bright yellow crystalline solid at room temperature. Up to 256 cash back Get the detailed answer.
Sulphur trioxide reacts with water to form sulphuric acid. Sulfur dioxide oxygen sulfur trioxide. 2 The Pressure is between 1 and 2 atmospheres.
Sulfur dioxide gas reacts with oxygen gas to form sulfur trioxide gas. Assuming that the temperature remains constant what is the final pressurea. Answer 1 of 3.
Sulfur can also combine with oxygen to produce sulfur trioxide. Science Chemistry QA Library Sulfur dioxide reacts with oxygen to form sulfur trioxide. Sulphur dioxide combines with oxygen to form sulphur trioxideii.
The reaction of Sulfur dioxide and oxygen react to form sulfur trioxide is as follows. An industrial chemist studying this reaction fills a 500. An industrial chemist studying this reaction fills a 500 ml flask with 29 atm of sulfur dioxide gas and 34 atm of oxygen gas and when the mixture has come to equilibrium measures the partial pressure of sulfur troxide gas to be 0.
Sulfur dioxide reacts with oxygen to form sulfur trioxide. D Sulfur dioxide is readily absorbed in the respiratory system where it is A soothing. Often the nonmetal reactants can combine in different ratios and produce different products.
2SO 2 g O 2 g 2SO. Sulfur particles dispersed in the air then react with oxygen to form sulfur dioxide as shown. Sulfur dioxide and oxygen react to form sulfur trioxide during one of the key steps in sulfuric acid synthesis.
Balance the equations for the above and draw the Lewis structures for each of the. 4m01 2 503 2m01 Cztlqt 5 Oz d. Sodium reacts with watera Write the balanced chemical equation for any one of the above reactionsb Which of the above reactions is a reversible reactionc What is the effect of pressure and temperature on this reversible reaction.
Again from the balanced equation the mole ratio of SO2 to O2 is. Sulfur reacts with oxygen to form sulfur dioxide. B a powerful irritant.
Industrially sulfur trioxide is an important precursor to sulfuric acid and is formed from the reaction between sulfur dioxide SO2 and oxygen gas O2 as shown in the chemical equation below. When nonmetals react with one another the product is a molecular compound. 2 SO3In a 100 L flask 200 atm of SO2 react with 500 atm of O2.
2SO2g O2g 2SO3g In a 100 L flask 200 atm of SO2 reacts wit. 2SO2 O2 2SO3 1. In the second stage sulfur dioxide reacts with more oxygen to make sulfur trioxide.
From the balance equation the mole ratio of SO2 to SO3 is 22 or simply 11. EqS_8 8O_2 rightarrow. An industrial chemist studying this reaction fills a 20L flask with 31 atm of sulfur dioxide gas and 16 atm of oxygen gas and when the mixture has come to equilibrium measures the partial pressure of sulfur trioxide gas to be 062.
Up to 256 cash back Get the detailed answer. A Sulfur trioxide reacts with water to form A sulfur dioxide. 8SO_2 eq The reaction above is an exothermic reaction.
Sulfur is the tenth most abundant element by mass in the universe. 3 A Vanadium oxide V 2 O 5 catalyst is used. Where P represents the pressure at equilibrium of each reactant.
Sulfur dioxide and oxygen react to form sulfur trioxide during one of the key steps in sulfuric acid synthesis. ML flask with. What combines with oxygen and sulfur dioxide.
Sulfur dioxide reacts with oxygen to form sulfur trioxide. The flask contained 00550 mol of sulfur dioxide and 00720 mol of sulfur trioxide at equilibrium. Sulfur dioxide oxygen sulfur trioxide.
Answer 1 of 9. Sulfur dioxide reacts with oxygen to form sulfur trioxide according to the equation 2SO2 g O2 g 2SO3 g Samples of sulfur dioxide oxygen and sulfur trioxide were added to a flask of volume 140 dm3 and allowed to reach equilibrium at a given. Sulfur dioxide reacts with oxygen to form sulfur trioxide according to the equation 2SO2g O2g 2SO3g Samples of sulfur dioxide oxygen and sulfur trioxide were added to a flask of volume 140 dm3 and allowed to reach equilibrium at a given temperature.
The sulfur dioxide is of particular concern because over time it will turn into sulfur trioxide which can react with water in the air and form acid. Sulphur reacts with oxygen to form sulphur trioxide through sulphur monoxide and sulphur dioxide. Catalyst vanadium pentoxide or platinum V2O5 is preferred Temp 450500 Pressure id around 100 atm Dont forget to upvote.
About 99 of the sulfur dioxide is converted into sulfur trioxide.
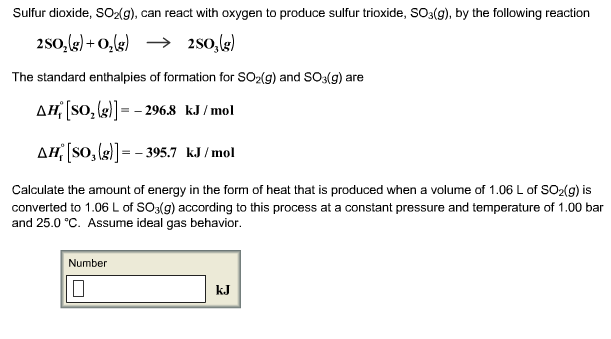
Solved Sulfur Dioxide So2g Can React With Oxygen To Chegg Com

Two Chemical Reactions Are Given I Sulphur Dioxide Combines With Oxygen To Form Sulphur Trioxide Ii Sodium Reacts With Water A Write The Balanced Chemical Equation For Any One Of The Above Reactions B Which

How To Balance So2 O2 So3 Youtube

Sulfur Dioxide Gas Reacts With Oxygen Gas To Form Sulfur Trioxide Gas Express Your Answer As A Brainly Com
0 Comments